Abstract:
Smoking has an adverse impact on several female reproductive life aspects, like oviducts function, uterus impairment, menstrual function disturbance. Moreover, smoking during pregnancy is a fundamental cause of health problems for both mother and fetus. It raises embryo implantation complexities associated with health conditions such as heart and cardiovascular diseases, pulmonary diseases, and hypertension. Smoking has an adverse impact on several female reproductive life aspects, like oviducts function, uterus impairment, menstrual function disturbance. Moreover, smoking during pregnancy is a fundamental cause of health problems for both mother and fetus. It raises embryo implantation complexities associated with health conditions such as heart and cardiovascular diseases, pulmonary diseases, and hypertension. Furthermore, several cigarette smoke compounds have toxic impacts on clinical IVF/ICSI treatment.

Smoking and female reproductive health
Smoking can cause a reduction in ovarian function and fertility.
Extensive population-based research indicates that smoking is correlated with earlier menopause and an increased risk of osteoporosis, indicating that smoking damages the ovarian function and decreases the monthly possibility of conception in the natural cycle. Moreover, smoking also reduces the opportunities for pregnancy in assisted reproduction cycles (VANVOORHIS et al., 1996). Smoking influences various aspects of the female reproductive function and, consequently, natural female fertility by using several differential impacts on multiple targets, such as the ovary, oviduct, and uterus Figure 1 (de Angelis et al., 2020) ,
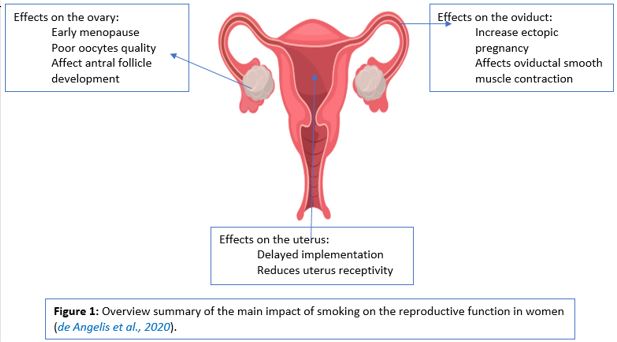
Uterus impairment by smoking
Smoking can affect the endometrium receptivity and trophoblast gene expression in the uterus. Uterine implantation requires a stable and tight interaction among a receptive endometrium and a competent blastocyst. A precisely and adequately timed remodelling of the endometrium is demanded before embryo arrival in the uterus. In addition, trophoblast gene expression can have a vital role in the endometrial lining interaction, and impaired trophoblast cannot succeed in implantation (de Angelis et al., 2020)
Blastocyst implantation starts with the attachment of the blastocyst to the endometrium, followed by an endometrial invasion of the trophoblast cells. The endometrium helps implantation through a discrete period (cycle days 19-21) in the mid-secretory phase of the cycle, known as the “window of implantation.” The endometrial glandular at the first stage changes into a secretory state, followed by numerous cytokines and growth factors production, facilitating implantation , such as CXCL12 (SDF1) and its receptor CXCR4 are applicable in numerous necessary processes in the implantation process and embryogenesis. (Sahin Ersoy et al., 2017) . Sahin Ersoy et al., 2017 evaluate CXCL12 and FGF2 as endometrial receptivity markers both in vivo and in vitro and the effects of smoking on the endometrial receptivity. CXCL12 and FGF2 mRNA expression were significantly decreased in the smoking group, where CXCL12 was reduced to 58% of control levels and FGF2 to 6% Figure 3 (Sahin Ersoy et al., 2017) .
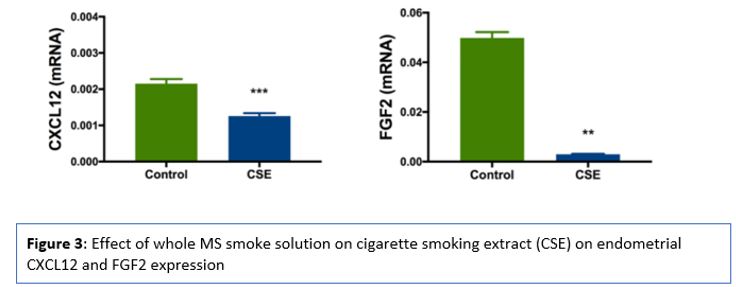
Uterus impairment by smoking
Smoking can affect the endometrium receptivity and trophoblast gene expression in the uterus. Uterine implantation requires a stable and tight interaction among a receptive endometrium and a competent blastocyst. A precisely and adequately timed remodelling of the endometrium is demanded before embryo arrival in the uterus. In addition, trophoblast gene expression can have a vital role in the endometrial lining interaction, and impaired trophoblast cannot succeed in implantation (de Angelis et al., 2020)
Blastocyst implantation starts with the attachment of the blastocyst to the endometrium, followed by an endometrial invasion of the trophoblast cells. The endometrium helps implantation through a discrete period (cycle days 19-21) in the mid-secretory phase of the cycle, known as the “window of implantation.” The endometrial glandular at the first stage changes into a secretory state, followed by numerous cytokines and growth factors production, facilitating implantation , such as CXCL12 (SDF1) and its receptor CXCR4 are applicable in numerous necessary processes in the implantation process and embryogenesis. (Sahin Ersoy et al., 2017) . Sahin Ersoy et al., 2017 evaluate CXCL12 and FGF2 as endometrial receptivity markers both in vivo and in vitro and the effects of smoking on the endometrial receptivity. CXCL12 and FGF2 mRNA expression were significantly decreased in the smoking group, where CXCL12 was reduced to 58% of control levels and FGF2 to 6% Figure 3 (Sahin Ersoy et al., 2017) .
Impact of smoking on fertility, embryo implementation, and life birth rate ( ART treatment )
Cigarette smoke components such as polycyclic aromatic hydrocarbons (PAHs) such as benzo(a)pyrene (BaP), nitrosamines, heavy metals, nicotine, aromatic amines and carbonyl compounds have several impacts on the female reproductive system. Nicotine shows to slow uterine decidualization, motility and migration of uterine endothelial cells in vitro (Heger, Sator, Walch and Pietrowski, 2018). Also, nicotine and BaP in cigarette smoke repress endometrial epithelial cell proliferation throughout a nitric oxide-mediated pathway in a dose and time-dependent manner. PAH derivatives modify cytochromes required in estrogen metabolism, pointing to a smoke-associated anti-estrogenic consequence. Cadmium (Cd) can decrease the size or complete loss of follicles, and damage cumulus expansion (Heger, Sator, Walch and Pietrowski, 2018) .
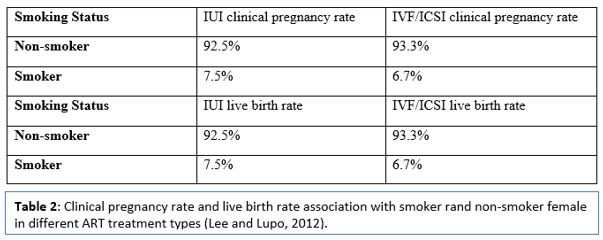
The impact of smoking on assisted reproduction technology has been evaluated in several types of research. An association between clinical pregnancy rate and smoking has been recorded, Lyngsø et al., 2020 reported a significant difference in the pregnancy rate of smoking and non-smoking female in all ART cycles, including IUI, IVF and ICSI (Lee and Lupo, 2012) (Table 2).
References
üAllen, A., Oncken, C. and Hatsukami, D., 2014. Women and Smoking: The Effect of Gender on the Epidemiology, Health Effects, and Cessation of Smoking. Current Addiction Reports, 1(1), pp.53-60.
üBouyer, J., 2003. Risk Factors for Ectopic Pregnancy: A Comprehensive Analysis Based on a Large Case-Control, Population-based Study in France. American Journal of Epidemiology, 157(3), pp.185-194.
üChen, C., Cho, S., Damokosh, A., Chen, D., Li, G., Wang, X. and Xu, X., 2000. Prospective study of exposure to environmental tobacco smoke and dysmenorrhea. Environmental Health Perspectives, 108(11), pp.1019-1022.
üde Angelis, C., Nardone, A., Garifalos, F., Pivonello, C., Sansone, A., Conforti, A., Di Dato, C., Sirico, F., Alviggi, C., Isidori, A., Colao, A. and Pivonello, R., 2020. Smoke, alcohol and drug addiction and female fertility. Reproductive Biology and Endocrinology, 18(1).
Authors:
Mustafa Zakaria1*, Aya Al-ibraheemi2 *, Wassym Senhaji3, Mohammed Ennaji4,Noureddine Louanjli5 ,Mohammed Zarqaoui6
ü1-Department Reproductive Biology, Northwestern University, Executive Committee President of the MSERM, Casablanca, Morocco
ü2-Department Embryologist, University of Nottingham, Nottingham, Member of the MSERM, United Kingdom
ü3-Department Gynaecology, University of Paris, Vice -Executive Committee President of the MSERM, Casablanca, Morocco
ü4-Department Embryologist, University of Cadi Ayyad in Marrakech, Member of the MSERM, Casablanca, Morocco
ü5-Departement of Clinical Biology, University of Strasbourg, Vice Chair of Scientific Committee of the MSERM, Casablanca, Morocco
ü6-Department Gynaecology, University of Lille, President of the MSERM, Casablanca, Morocco
