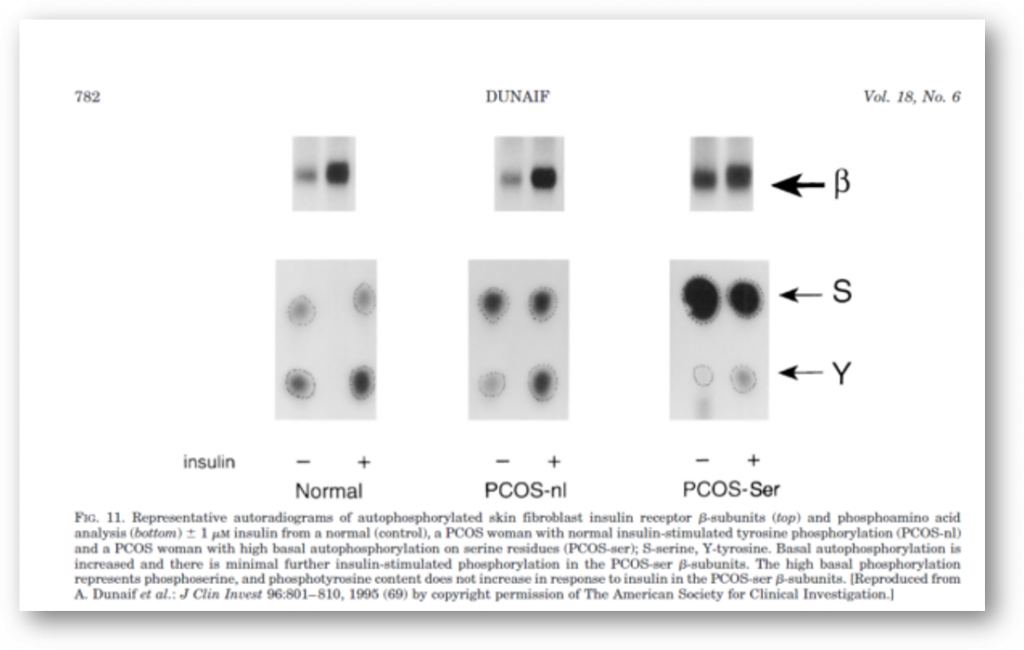
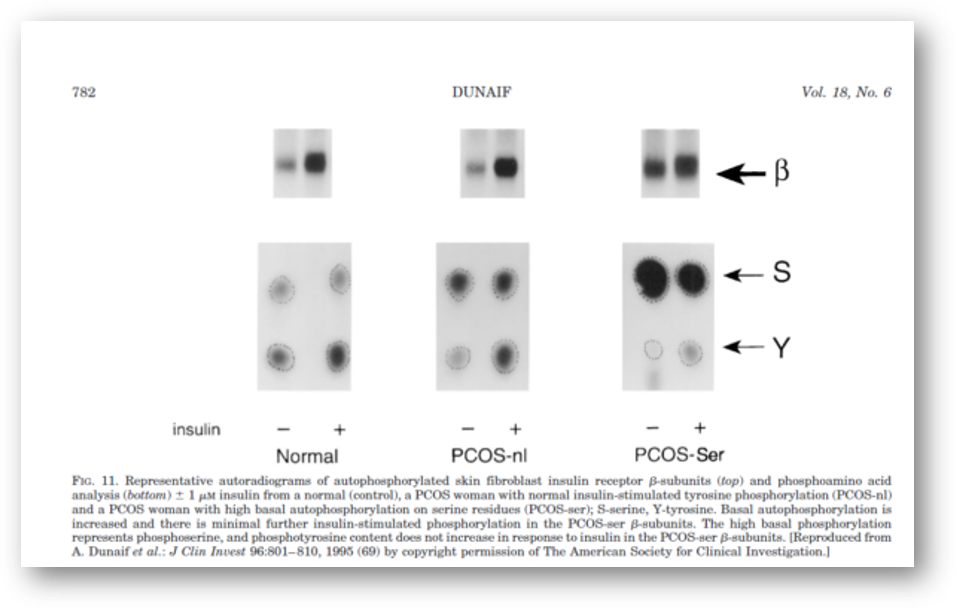
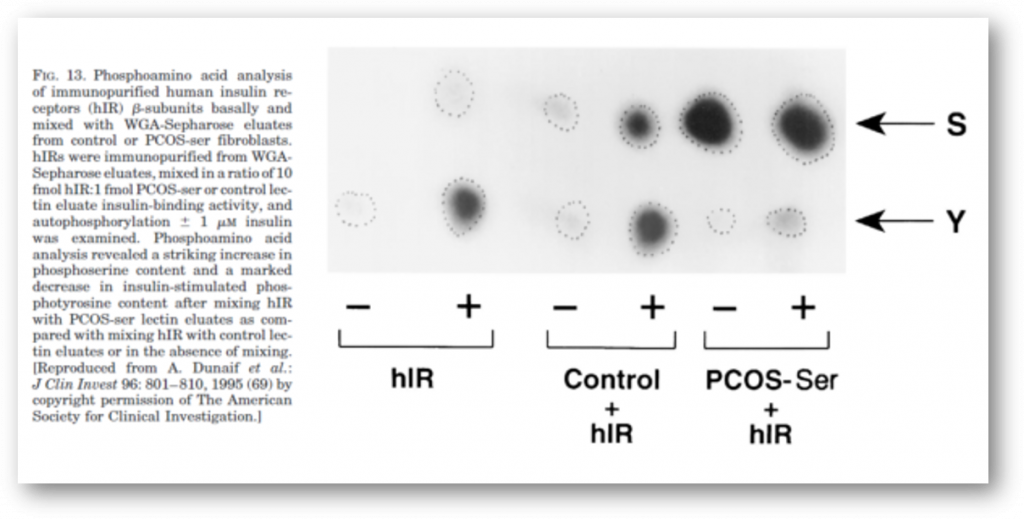
It is now clear that PCOS is often associated with profound insulin resistance as well as with defects in insulin secretion. These abnormalities, together with obesity, explain the substantially increased prevalence of glucose intolerance in PCOS. Moreover, since PCOS is an extremely common disorder, PCOS-related insulin resistance is an important cause of NIDDM in women (Table 3). The insulin resistance in at least 50% of PCOS women appears to be related to excessive serine phosphorylation of the insulin receptor. A factor extrinsic to the insulin receptor, presumably a serine/threonine kinase, causes this abnormality and is an example of an important new mechanism for human insulin resistance related to factors controlling insulin receptor signaling. Serine phosphorylation appears to modulate the activity of the key regulatory enzyme of androgen biosynthesis, P450c17.
It is thus possible that a single defect produces both the insulin resistance and the hyperandrogenism in some PCOS women (Fig. 19). Recent studies strongly suggest that insulin is acting through its own receptor (rather than the IGF-I receptor) in PCOS to augment not only ovarian and adrenal steroidogenesis but also pituitary LH release. Indeed, the defect in insulin action appears to be selective, affecting glucose metabolism but not cell growth. Since PCOS usually has a menarchal age of onset, this makes it a particularly appropriate disorder in which to examine the ontogeny of defects in carbohydrate metabolism and for ascertaining large three-generation kindreds for positional cloning studies to identify NIDDM genes. Although the presence of lipid abnormalities, dysfibrinolysis, and insulin resistance would be predicted to place PCOS women at high risk for cardiovascular disease, appropriate prospective studies are necessary to directly assess this.
Insulin action in vivo in PCOSAlthough insulin has a number of actions, in addition tothose regulating glucose metabolism, such as inhibition oflipolysis and stimulation of amino acid transport (51), theeffects of insulin on glucose metabolism are usually exam-ined in studies of insulin resistance (60). This can be studiedquantitatively in humans with the euglycemic glucose clamptechnique: a desired dose of insulin is administered andeuglycemia is maintained by a simultaneous variable glucoseinfusion whose rate is adjusted based on frequent arterial-ized blood glucose determinations and a negative feedbackprinciple (60 – 62). At steady state, the amount of glucose thatis infused equals the amount of glucose taken up by theperipheral tissues and can be used as a measure of peripheralsensitivity to insulin, known as insulin-mediated glucosedisposal (IMGD) or M (61, 62). The suppression of hepaticglucose production by insulin can be assessed by the use ofa simultaneous infusion of isotopically labeled glucose.Insulin-mediated glucose disposal occurs only in muscle(skeletal and cardiac) and in fat; muscle accounts for about85% of this (60)

Hypotheses Explaining the Association of InsulinResistance and PCOSA. Causal association1. Do androgens cause insulin resistance?If glucose utilizationis expressed as a function of muscle mass rather than totalbody mass, women do appear to be more insulin sensitivethan men (158, 159). Moreover, when isolated fat cells arecompared, female adipocytes are more sensitive than maleadipocytes to insulin-mediated glucose uptake (160). Theseare subtle differences, however, and do not approach thedegree of impairment in insulin sensitivity observed in PCOS(54, 55). Finally, in the rare syndromes of extreme insulinresistance and hyperandrogenism, specific molecular defectsin insulin action have been clearly identified as the cause ofinsulin resistance (19, 161).It is possible, however, that androgens may produce mildinsulin resistance. Women receiving oral contraceptives con-taining “androgenic” progestins can experience decompen-sations in glucose tolerance, as can individuals receivingsynthetic anabolic steroids



References
1.Dunaif A, Givens JR, Haseltine F, Merriam GR (eds)1992 ThePolycystic Ovary Syndrome. Blackwell Scientific, Cambridge, MA2.Franks S1995 Polycystic ovary syndrome. N Engl J Med 333:853–8613.Polson DW, Wadsworth J, Adams J, Franks S1988 Polycysticovaries — a common finding in normal women. Lancet 1:870 – 8724.Pettersson F, Fries H, Nillius SJ1973 Epidemiology of secondaryamenorrhea. Am J Obstet Gynecol 117:80 – 865.Hull MGR1987 Epidemiology of infertility and polycystic ovariandisease: endocrinological and demographic studies. Gynecol En-docrinol 1:235–2456.Goldzieher JW, Green JA1962 The polycystic ovary. I. Clinical andhistologic features. J Clin Endocrinol Metab 22:325–3387.Burghen GA, Givens JR, Kitabchi AE1980 Correlation of hy-perandrogenism with hyperinsulinism in polycystic ovarian dis-ease. J Clin Endocrinol Metab 50:113–1168.Dunaif A, Hoffman AR1988 Insulin resistance and hyperandro-genism: clinical syndromes and possible mechanisms. In: PancheriP, Zichella L (eds) Biorhythms and Stress in the Physiopathologyof Reproduction. Hemisphere Publishing Co, Washington, DC, pp293–3179.Barbieri RL, Ryan KJ1983 Hyperandrogenism, insulin resistance,and acanthosis nigricans syndrome: a common endocrinopathywith distinct pathophysiologic features. Am J Obstet Gynecol147:9010.Poretsky L, Kalin MF1987 The gonadotropic function of insulin.Endocr Rev 8:132–14111.Poretsky L1991 On the paradox of insulin-induced hyperandro-genism in insulin-resistant states. Endocr Rev 12:3–1312.Ehrmann DA, Barnes RB, Rosenfield RL1995 Polycystic ovarysyndrome as a form of functional ovarian hyperandrogenism dueto dysregulation of androgen secretion.
Endocr Rev 16:322–35313.Crowley WF1996 New tools for pituitary-gonadal assessment. In:Filicori M, Flagmini C (eds) The Ovary: Regulation, Dysfunctionand Treatment. Elsevier, Amsterdam, pp 287–29314.Achard C, Thiers J1921 Le virilisme pilaire et son association al’insuffisance glycolytique (diabete des femmes a barb). Bull AcadNatl Med 86:51– 6415.Kierland RR, Lakatos I, Szijarto L1947 Acanthosis nigricans: Ananalysis of data in twenty-two cases and a study of its frequencyin necropsy material. J Invest Dermatol 9:299 –30516.Brown J, Winkelmann RK1968 Acanthosis nigricans: a study of90 cases. Medicine 47:33–5117.Barnes ND, Palumbo PJ, Hayles AM, Folgar H1974 Insulin re-sistance, skin changes and virilization: a recessively inherited syn-drome possibly due to pineal gland dysfunction. Diabetologia 10:284 –28918.Colle M, Doyard P, Chaussain J, Battin J, Job J1979 Acanthosisnigricans, hirsutisme et diabete insulin-resistant. Arch Fr Pediatr36:518 –52319.Taylor SI, Cama A, Accili D, Barbetti F, Quon J, Sierra MDLL,Suzuki Y, Koller E, Levy-Toledano R, Wertheimer E, MoncadaVY, Kadowaki H, Kadowaki T1992 Mutations in the insulin re-ceptor gene. Endocr Rev 13:566 –59520.Dunaif A1992 Insulin resistance and ovarian hyperandrogenism.The Endocrinologist 2:248 –26021.Kahn CR, Flier JS, Bar RS, Archer JA, Gorden P, Martin MM, RothJ1976 The syndromes of insulin resistance and acanthosis nigri-cans. N Engl J Med 294:739 –74522.Flier JS, Kahn R, Roth J, Bar RS1975 Antibodies that impair insulinreceptor binding in an unusual diabetic syndrome with severeinsulin resistance. Science 190:63– 65
ANDREA DUNAIF
PMID: 9408743
DOI: 10.1210/edrv.18.6.0318

